
Unigloves Cross Guard Antimicrobial Nitrile Glove
Unigloves Cross Guard Antimicrobial Nitrile Glove
(13)





Features You'll Love
Fingertips · Textured
Describes the surface texture of the fingertip area, affecting grip strength and tactile sensitivity during use.
Describes the surface texture of the fingertip area, affecting grip strength and tactile sensitivity during use.
Describes the surface texture of the fingertip area, affecting grip strength and tactile sensitivity during use.

Finger Thickness · 0.09 mm
Measures the material thickness at the fingertips, affecting tactile sensitivity, dexterity, and protection level during use.
Measures the material thickness at the fingertips, affecting tactile sensitivity, dexterity, and protection level during use.
Measures the material thickness at the fingertips, affecting tactile sensitivity, dexterity, and protection level during use.
Unigloves
Cross Guard Antimicrobial Nitrile Glove, 10 x 100 pcs
(13)
59,86 €
Price per 10 packages (1 000 pcs)
5,99 € / 100 pcs
Shipping fee is 7,95 € for orders under 600,00 €
Features You'll Love

Fingertips · Textured
Describes the surface texture of the fingertip area, affecting grip strength and tactile sensitivity during use.
Describes the surface texture of the fingertip area, affecting grip strength and tactile sensitivity during use.
Describes the surface texture of the fingertip area, affecting grip strength and tactile sensitivity during use.

Finger Thickness · 0.09 mm
Measures the material thickness at the fingertips, affecting tactile sensitivity, dexterity, and protection level during use.
Measures the material thickness at the fingertips, affecting tactile sensitivity, dexterity, and protection level during use.
Measures the material thickness at the fingertips, affecting tactile sensitivity, dexterity, and protection level during use.
Product description
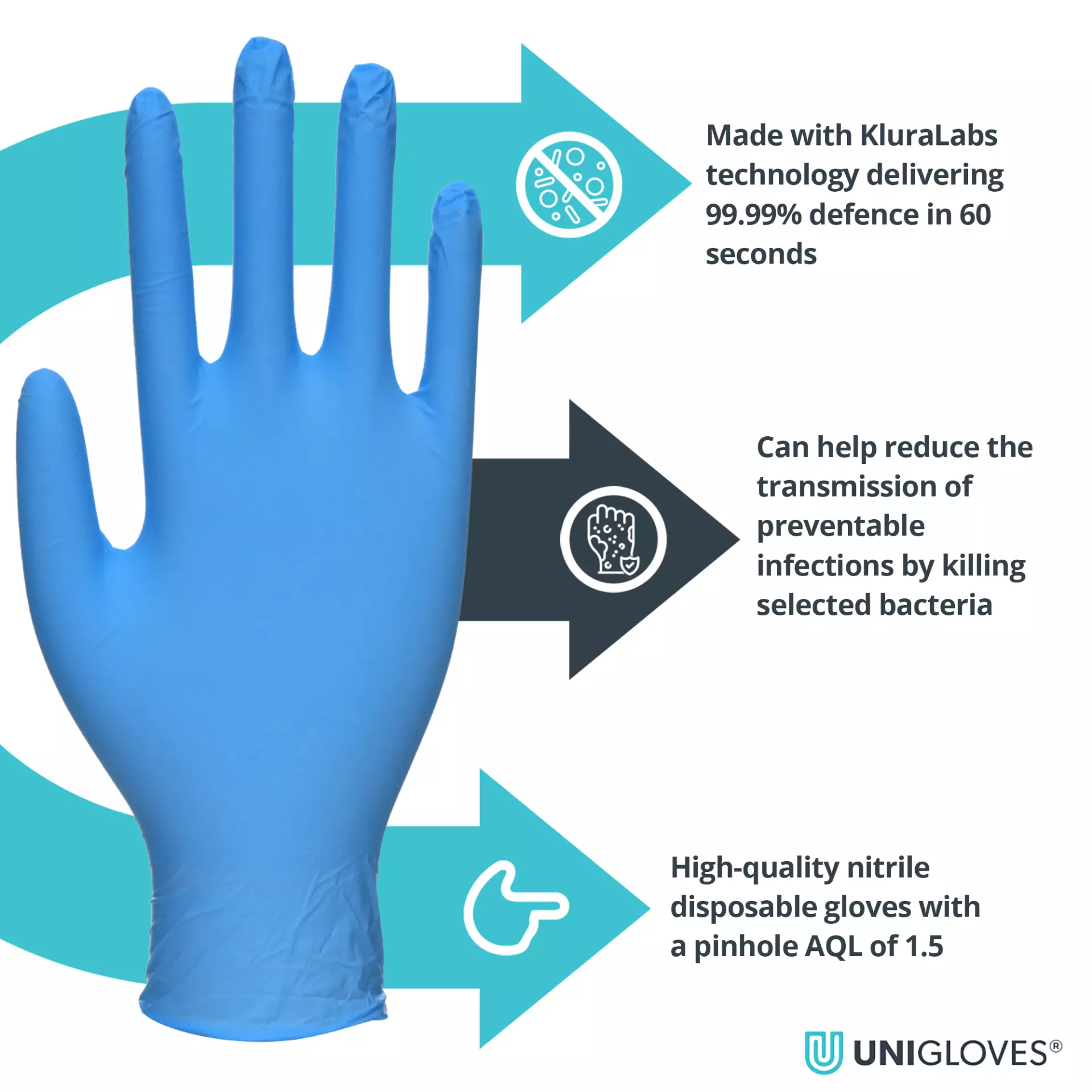
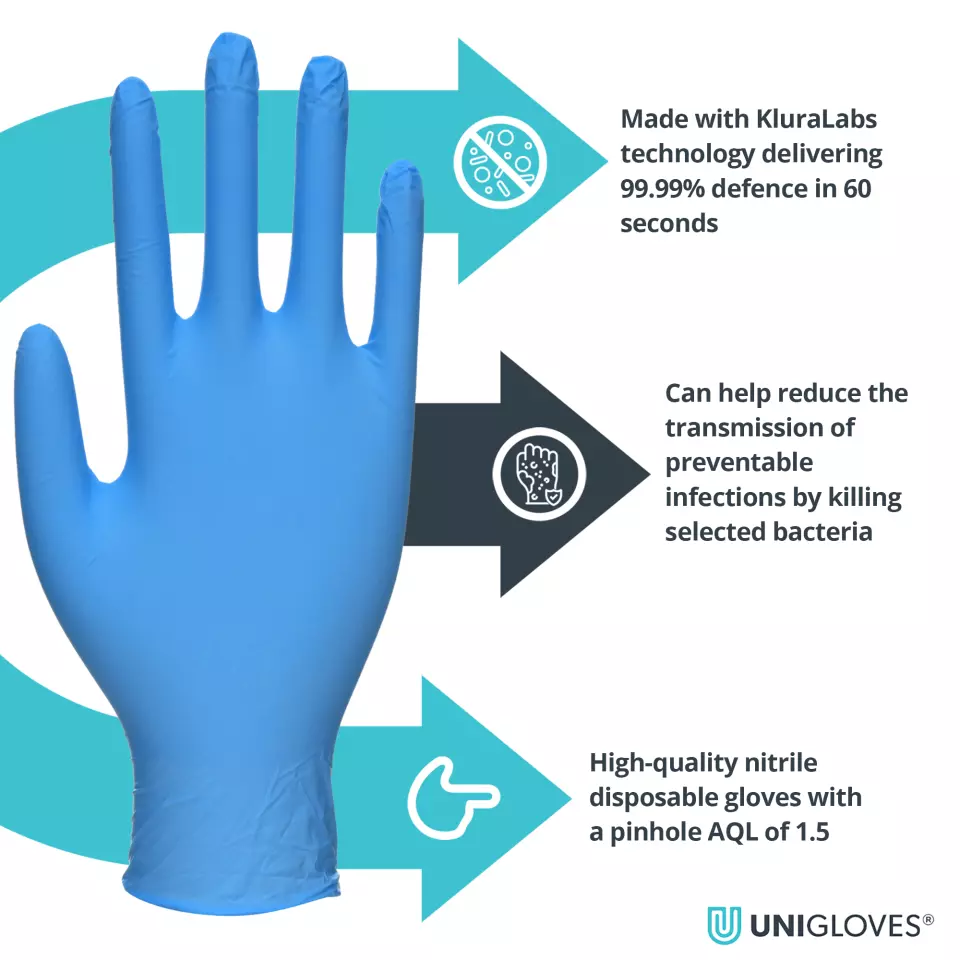
Eliminates 99.99% of selected bacteria in 60 seconds Can help reduce the transmission of preventable infections by killing harmful bacteria Class 1 Medical Device in Europe Third party ASTM Efficacy Validation Effective against Healthcare Acquired Infections No active ingredients and non leaching Suitable for contact with all food types
Eliminates 99.99% of selected bacteria in 60 seconds Can help reduce the transmission of preventable infections by killing harmful bacteria Class 1 Medical Device in Europe Third party ASTM Efficacy Validation Effective against Healthcare Acquired Infections No active ingredients and non leaching Suitable for contact with all food types
Eliminates 99.99% of selected bacteria in 60 seconds Can help reduce the transmission of preventable infections by killing harmful bacteria Class 1 Medical Device in Europe Third party ASTM Efficacy Validation Effective against Healthcare Acquired Infections No active ingredients and non leaching Suitable for contact with all food types
Describes the surface texture of the fingertip area, affecting grip strength and tactile sensitivity during use.
Phthalate-free for reduced chemical exposure, offering a safer choice for you and the items you handle.
Effortlessly use touch screens without removing your gloves, ensuring continuous protection and hygiene.
Your go-to gloves for diverse tasks, offering reliable protection and convenience across many applications.
Provides comfort and peace of mind, free from natural rubber latex to protect sensitive skin.
Complete finger coverage ensures full protection and hygiene for your hands, maintaining dexterity for all tasks.
Easily wear on either hand, ensuring quick protection and less waste.
Indicates whether gloves contain powder on the interior surface to aid donning, affecting ease of use and contamination control requirements.
The texture of the glove exterior, affecting grip strength and handling capability for different tasks and working conditions.
The visual appearance of the glove material, ranging from basic colors to specialized options for different professional and aesthetic preferences.
The base substance used to manufacture the glove, affecting chemical resistance, durability, flexibility, and compatibility with specific applications.
Measures the material thickness at the fingertips, affecting tactile sensitivity, dexterity, and protection level during use.
Measures how far the glove extends up the wrist and forearm, determining the level of coverage and protection provided during use.
Measures the material thickness at the palm area, affecting protection level and tactile sensitivity during use.
Indicates the maximum percentage of defective gloves acceptable in quality testing, with lower numbers representing higher quality standards.
Indicates how long gloves maintain their protective properties and quality when stored properly, typically measured in years from manufacture date.
- Chemical Resistance
- Hand Protection
Gloves with the EN 374-5:2016 rating are tested for resistance to penetration by bacteria and fungi, and potentially viruses. This means the gloves provide a protective barrier against microorganisms, helping to keep your hands safe from harmful biological agents.
Test results
This product protects you from bacteria and fungi. It passed leakage tests, making it a reliable barrier when handling materials where these micro-organisms are a risk, such as in cleaning or laboratory work.
This glove is tested to protect you from bacteria, fungi, and viruses. It provides a reliable barrier, having passed specific tests to ensure no leakage when exposed to these micro-organisms, making it suitable for handling contaminated materials.
Protective gloves with the EN 374-4:2013 rating are tested for their resistance to degradation by chemicals. This measures changes in the glove material, like swelling or hardening, ensuring the glove maintains its protective qualities when exposed to hazardous substances.
Test results
This glove's material resists breaking down when in contact with specific chemicals. This helps protect you from physical changes to the glove like swelling, cracking, or softening that could compromise your safety during use.
Gloves with the EN ISO 374-1:2016 rating are tested for resistance against dangerous chemicals and microorganisms, including penetration, permeation, and degradation. This means you can choose gloves designed to protect your hands from specific chemical hazards, ensuring safer use in various tasks.
Test results
Provides splash protection against at least one specific chemical for more than 10 minutes. These gloves are suitable for tasks involving limited or short-duration contact with specified low-risk chemicals.
Gloves with the EN 374-2 rating are tested for resistance to penetration by liquids and microorganisms. This means the glove material is checked for physical defects like pinholes or leaks, ensuring it forms a barrier against harmful substances. You can trust these gloves to be waterproof and block microorganisms.
Single-use medical gloves with the EN 455-3 rating are tested for biological safety, including potential chemical residues, latex proteins, and powder content. This ensures the gloves are safe for skin contact, minimizing the risk of irritation or allergic reactions for users and patients.
Medical gloves with the EN 455-2 rating are tested for physical properties like dimensions and strength, including force at break both before and after aging. This ensures the gloves fit correctly, are durable, and provide reliable protection against cross-contamination for both patient and user.
Medical gloves with the EN 455-1 rating are tested for freedom from holes to ensure they act as an effective barrier. This means the gloves reliably protect users and others from the transmission of microorganisms and contamination.
Medical gloves with the EN 455-4 rating are tested for their shelf life, ensuring they maintain critical properties like strength and barrier integrity over time. This means you can trust that the gloves will remain effective and safe to use until their expiration date, providing reliable protection when needed.
Food safe refers to the safety of food products that are used or consumed by people. In Europe, food safety is regulated by the European Union (EU) and the European Food Safety Authority (EFSA). These organizations set standards and requirements for food products to ensure they are safe to eat. To be considered "food safe" in Europe, a product must meet these standards and be free of harmful substances. This includes being free of harmful bacteria, pesticides, and other contaminants. Food products that do not meet these standards cannot be sold or used in the EU.
MD stands for "Medical Device." It refers to any instruments, apparatus, machines, implants, or other similar or related articles that are intended to be used for human beings for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease, injury or disability. In Europe, a MD Label is a special label that must be on all Medical Devices that are sold or used in the European Union (EU). The label must include information about the product, such as the name of the manufacturer, the intended use of the product and CE mark. To be able to sell or use a Medical Device in the EU, the device must meet certain standards and requirements set by the European Union and notified body.
Free delivery when you order more than 600,00 € from Unigloves
Supplier shipping fee 7,95 €
Brand minimum 0,00 €