Product description
The innovative COATS® (Colloidal Oatmeal System) nitrile gloves feature a specialized inner barrier designed to reduce skin damage and promote healing. The advanced inner protective layer contains colloidal oatmeal that moisturizes and rehydrates skin while relieving inflammation, irritation, and itching. These powder-free gloves combine superior protection with therapeutic benefits, making them ideal for extended wear in demanding environments.
Product Features:
- COATS® inner barrier technology
- Textured fingertips for optimum grip
- Powder-free construction
- Tight-fitting design
- Waterproof protection
- Chemical resistance protection: —40% Sodium Hydroxide —30% Hydrogen Peroxide —37% Formaldehyde
Technical Details:
- Material: Nitrile
- Thickness: 0.07 ± 0.02 mm (palm)
- Weight: 3.2 ± 0.3 g
- AQL Level: 1.5
Recommended Applications:
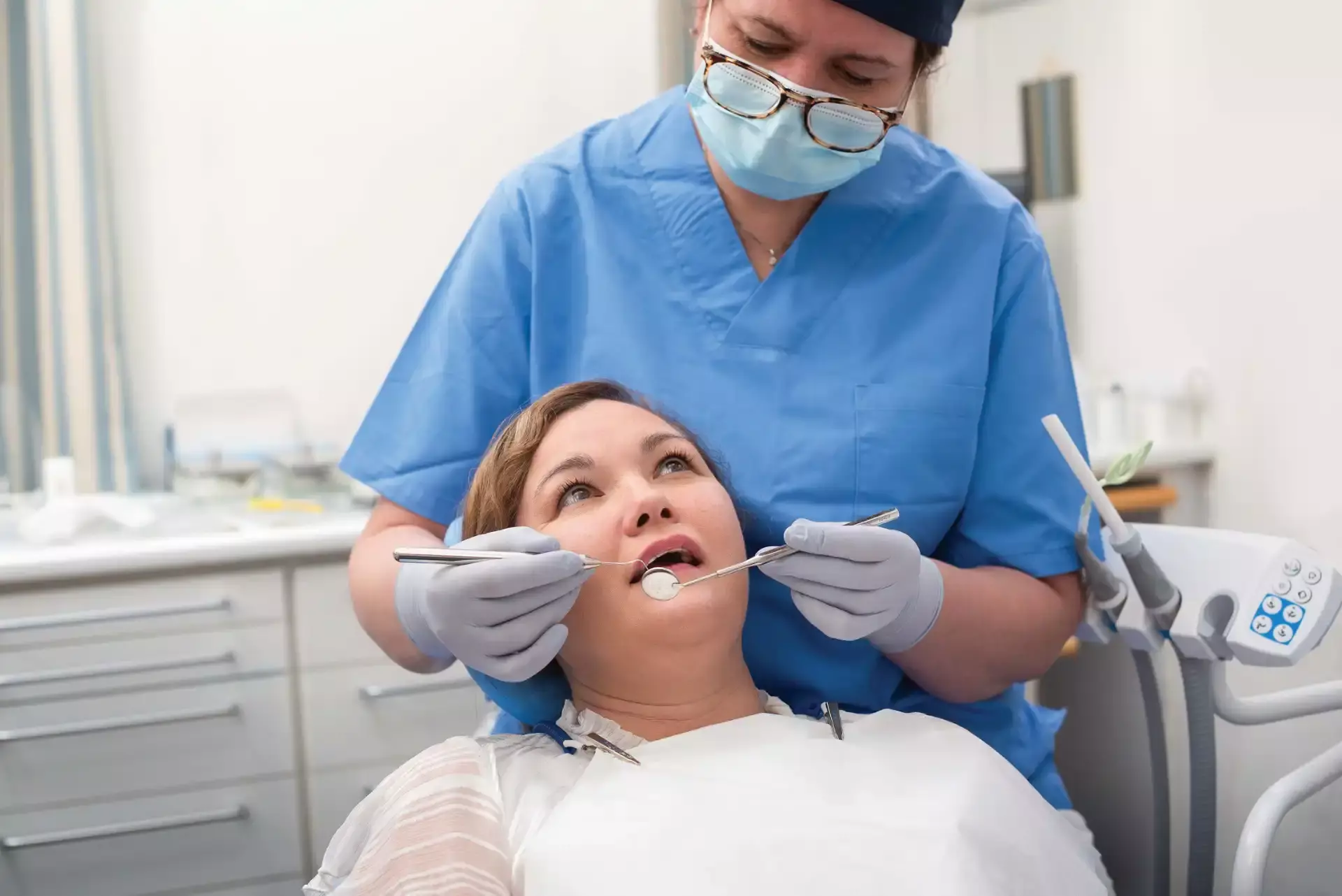
- Healthcare and Dental
- Beauty Industry and Cosmetology
- Food Processing
- Laboratory Work
Standards:
- CE Category III (Notified body 2777)
- EN ISO 21420:2020
- EN 455
- MDR Certified
- ASTM D6978-05 for Chemotherapy Drug Permeation
- Approved for direct food contact
About Disposable Nitrile Gloves
Disposable Nitrile Gloves provide latex-free hand protection with superior chemical and puncture resistance. These single-use gloves offer excellent tactile sensitivity and a secure fit, making them ideal for healthcare, food service, cleaning, and industrial applications where hygiene and safety are essential.
- Chemical Resistance
- Slip Resistant
- Antimicrobial Protection
- Water Resistance
Standards and labels
Granberg delivery terms
Free delivery when you order more than 750,00 € from Granberg
Supplier shipping fee 7,95 €
Brand minimum 500,00 €
61,46 €
Price per 10 packages (1 000 pcs)
6,15 € / 100 pcs
Shipping fee is 7,95 € for orders under 750,00 €