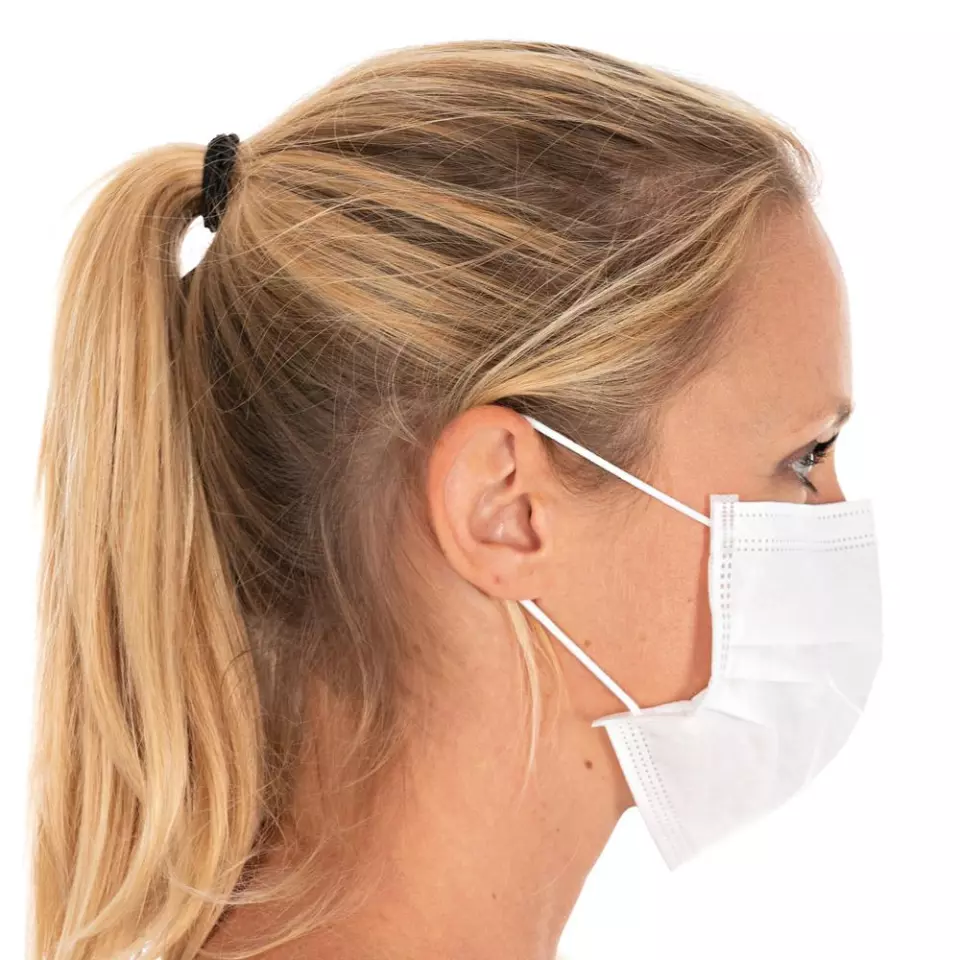
Hygostar IIR PP Face Masks, 3-ply, White, Made in Germany
Franz Mensch Nordics
visit storeProduct description
The product description has not been specified
The visual appearance of the respirator, which may affect workplace compliance, visibility requirements, and professional appearance.
Determines the physical form of the mask, affecting comfort, breathing space, storage convenience, and how well it seals against your face.
Determines the coverage area and design of the respirator, such as full face or half face, affecting protection level and compatibility with other safety equipment.
Measures the percentage of bacteria-sized particles filtered out, indicating the respirator's effectiveness at blocking biological contaminants.
Indicates the level and type of filtration provided, determining what contaminants the respirator protects against and the percentage of particles filtered.
The method used to secure the respirator to your face, affecting comfort, fit, and ease of putting on and removing the mask.
- Respiratory Protection
- Medical Protection
- Antimicrobial Protection
Request a free sample
Test first and buy later. Visit any product page to request your free sample.
Standards and labels
EN 14683 is a European standard for face masks. It includes requirements for mask design, testing, and labeling. Test results include things like filtration efficiency and breathability. The standard also includes requirements for packaging and labeling. The 2019 version updates the previous 2014 version.
Test results
Bacterial Filtration Type IIRThe Bacterial Filtration Efficiency (BFE) result for Type IIR in the standard EN 14683:2019 assesses the efficacy of medical face masks in preventing the passage of aerosolized bacteria. This is crucial for medical environments to ensure a high level of protection against potential bacterial infections transmissible through human respiratory droplets. For Type IIR under EN 14683:2019, the bacterial filtration efficiency test involves determining the percentage of bacteria-laden aerosols that do not pass through the mask material. Masks must demonstrate a BFE of ≥98% at a specified bacteria challenge load, ensuring superior filtration capabilities. This higher requirement reflects Type IIR's application in environments where exposure to blood and bodily fluids is possible, granting them suitability for surgeries and other medical proceedings where stringent sterile conditions are essential.
"Made in Germany" is a label used to indicate that a product has been manufactured or produced in Germany. The label is not regulated by EU laws and doesn't have specific requirements, but it's an indication of quality, craftsmanship and reliability that many German companies and products are known for. However, some German companies and industries may have their own standards and certifications for the "Made in Germany" label, such as VDA for the automobile industry or TUV for the electronic products. In addition, the German government does not have any specific laws or regulations for the use of "Made in Germany" label. It is up to the companies themselves to decide if they want to use it, and if they do, it is their responsibility to ensure that the products are truly made in Germany.
Food safe refers to the safety of food products that are used or consumed by people. In Europe, food safety is regulated by the European Union (EU) and the European Food Safety Authority (EFSA). These organizations set standards and requirements for food products to ensure they are safe to eat. To be considered "food safe" in Europe, a product must meet these standards and be free of harmful substances. This includes being free of harmful bacteria, pesticides, and other contaminants. Food products that do not meet these standards cannot be sold or used in the EU.
CE Marking is a label that shows a product meets certain safety and environmental standards set by the European Union. To get the CE Marking, a company must test and certify their product meets these standards. CE Marking is required for many products sold in the EU, including electronics, machinery, toys and medical devices. It helps ensure that products are safe for consumers and the environment, and allows for easy trade within the EU.
MD stands for "Medical Device." It refers to any instruments, apparatus, machines, implants, or other similar or related articles that are intended to be used for human beings for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease, injury or disability. In Europe, a MD Label is a special label that must be on all Medical Devices that are sold or used in the European Union (EU). The label must include information about the product, such as the name of the manufacturer, the intended use of the product and CE mark. To be able to sell or use a Medical Device in the EU, the device must meet certain standards and requirements set by the European Union and notified body.
Franz Mensch Nordics delivery terms
Free delivery for all Franz Mensch Nordics products
1 774,86 kr
Price per 60 packages (3 000 pcs)
0,59 kr / piece
Free delivery
A carton contains 60 packages (3 000 pieces)
Need larger quantities?
Other products you may like
Recently viewed
Need help?
Get help from our experts
Other products you may like
Similar products you may like
Autonomous sourcing platform
The most efficient way to source and order supplies for your operations
Sourcing
Ordering
List products you’re looking for and we’ll find the best products and prices for you – all for free.
Need help?
Get help from our experts