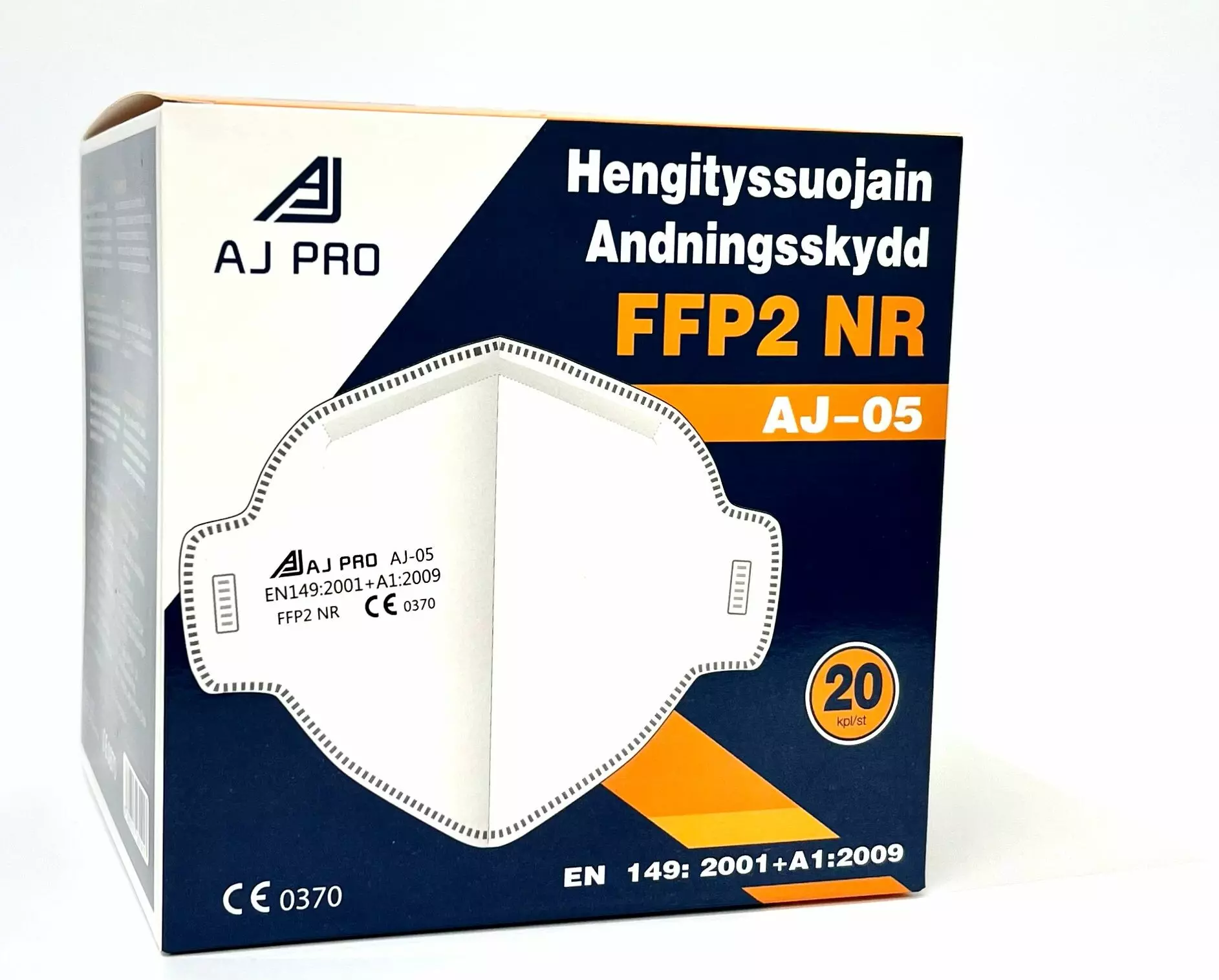
Aj Pro FFP2 with Neck Strap
4.8 / 5
Product description
The product description has not been specified
About Respirator
Respirators are specialized protective masks designed to filter harmful airborne particles, providing reliable breathing protection in hazardous environments. With secure facial seals and various filtration ratings, these disposable devices are essential for workers in construction, healthcare, and industrial settings where air quality is compromised.
- Medical Protection
- Respiratory Protection
Standards and labels
Elers Medical delivery terms
Free delivery for all Elers Medical products
Prices excl. VAT
1 815,73 kr
Price per 40 packages (800 pcs)
2,27 kr / piece
Estimated delivery: Fri Sep 19 - Mon Sep 22
Minimum: 1 carton
Add 1 to reach minimum
Free delivery
A carton contains 40 packages (800 pieces)
View packaging details