Loading Semperguard for Germany
Change the country of delivery or ask for an alternative for product code NRG-XLITE100 by contacting one of our product experts.
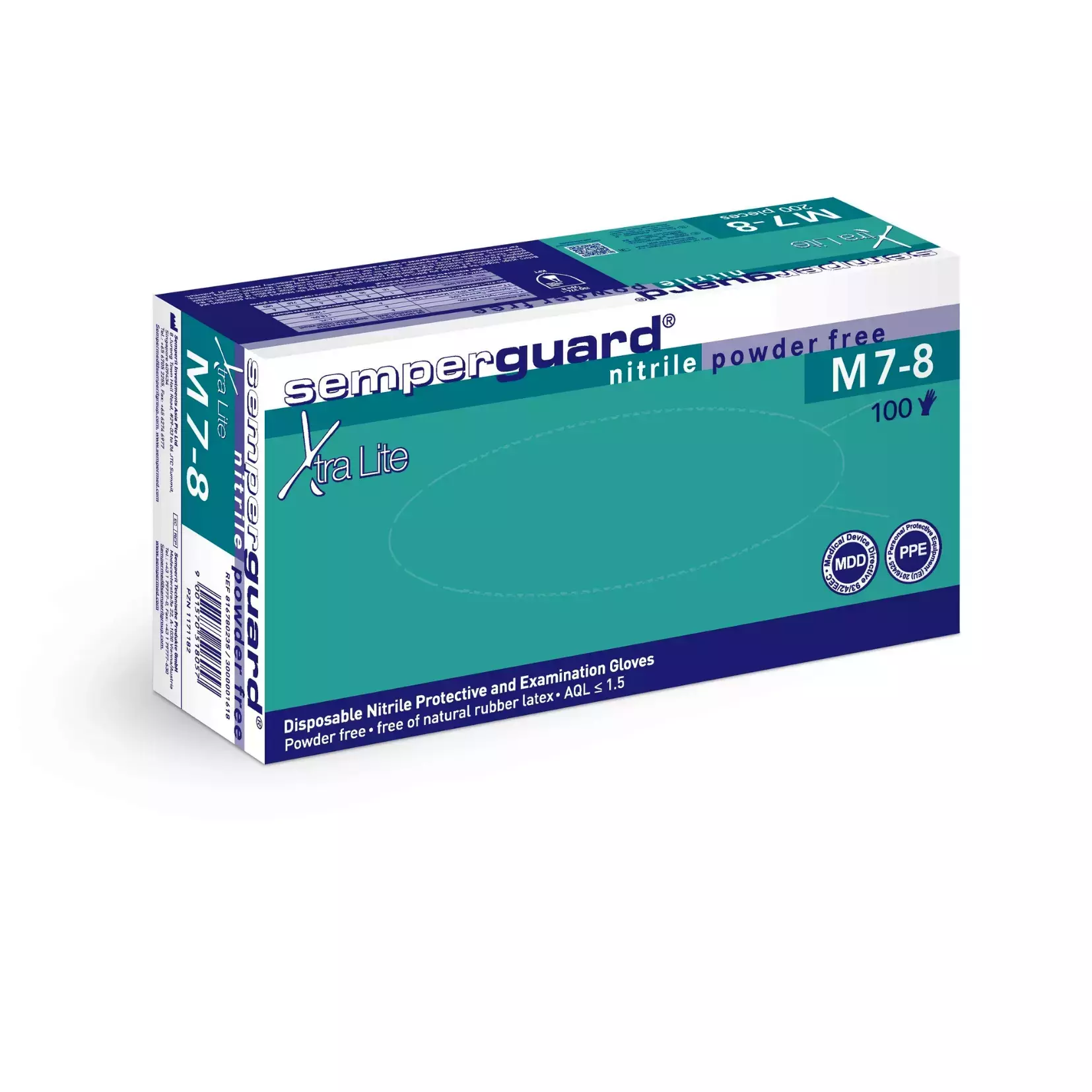
Semperguard Nitrile Gloves
Semperguard Nitrile Gloves
(17)
Change the country of delivery or ask for an alternative for product code NRG-XLITE100 by contacting one of our product experts.
Get help from our experts
You can pay with any of the following popular payment methods:
Get in touch with our customer support if you need help
Get help from our experts