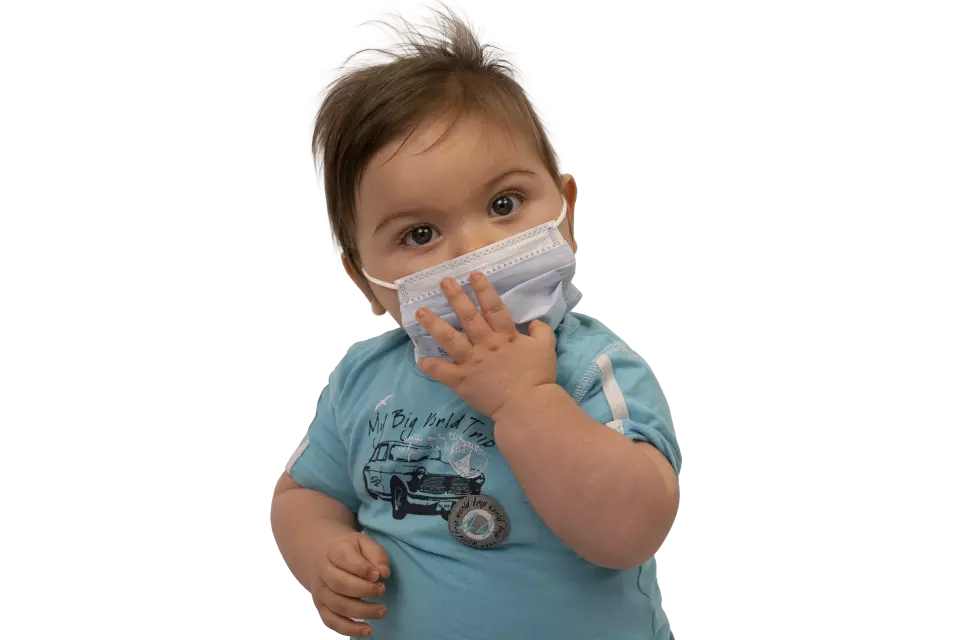
Kolmi Softex Mask with Earloops, Type II Kids, Blue
Medicom
visit storeProduct description
This pediatric medical mask is specifically designed for children aged 1 to 5 years, featuring exclusive Origami folding and Smile welding technology for optimal facial fit and comfort. The hypoallergenic construction includes a soft Softex inner layer that's gentle on sensitive skin, while the polypropylene material effectively protects the environment from droplets emitted by the child.
Product Features:
- Exclusive Origami folding and Smile welding for enhanced fit
- Softex inner layer for non-irritating comfort
- Hypoallergenic materials
- Designed specifically for children aged 1-5 years
- Durable construction for prolonged use
Technical Details:
- Material: Polypropylene
- Non-irritating inner layer
- Suitable for sensitive skin
Standards:
- EU Regulation 2017/745 on Medical Devices
- MD Class I certification
- CE marked
- Medical mask type II classification
Offers a snug, adaptable fit due to its inherent stretch. Conforms comfortably to the head, ensuring secure placement for reliable respiratory protection.
Easy to use and quick to put on, earloops offer a convenient fit. Ideal for applications requiring frequent donning and doffing of the respirator.
Defines the mask's coverage area (full face or half face), affecting protection level, visibility, and compatibility with other safety equipment.
Defines the structural design of the respirator (cup, flat, or fold), affecting facial fit, seal effectiveness, comfort during extended wear, and storage convenience.
Indicates the filtration standard and hazard protection level, from basic particle filtering (P1/FFP1) to high-efficiency gas and vapor protection (A2, B2, etc.).
Indicates the exterior hue of the respirator, which may signify specific protection types, enhance visibility in workplaces, or meet industry color-coding standards.
- Clean Room
- Respiratory Protection
- Medical Protection
- Antimicrobial Protection
Request a free sample
Test first and buy later. Visit any product page to request your free sample.
Standards and labels
EN 14683 is a European standard for face masks. It includes requirements for mask design, testing, and labeling. Test results include things like filtration efficiency and breathability. The standard also includes requirements for packaging and labeling. The 2019 version updates the previous 2014 version.
Test results
Bacterial Filtration Type IIThe Bacterial Filtration result for Type II in the EN 14683:2019 standard specifies a minimum bacterial filtration efficiency (BFE). The standard requires that Type II medical face masks must achieve at least a 98% BFE, which means that they are capable of filtering out at least 98% of the bacteria present in the testing aerosol. This is crucial for ensuring a high level of protection against the transmission of bacteria through respiratory droplets.
EN ISO 11737-1:2018/A1:2021 is a standard that defines how to test the sterilization of medical devices. It includes requirements for the methods used to sterilize the device and how to test to make sure the sterilization process was successful. Test results should show that the device is free from harmful microorganisms and safe for use. It was amended in 2021 which is why it has A1 at the end of the name.
Test results
Service Reliability PassedEN ISO 9001:2015 is a globally recognized standard that specifies requirements for a quality management system (QMS), focusing on numerous aspects of quality management in organizations, aiming to enhance customer satisfaction through the effective application of the system. The 'Service Reliability' with a 'Passed' designation indicates that an organization has successfully demonstrated its ability to consistently provide services that meet customer and regulatory requirements while aiming for continual improvement. This assessment involves evaluating various elements of the QMS including service planning, execution, and monitoring, to ensure reliability and performance consistency. The practical implications for organizations that pass this aspect of the standard are significant; it establishes them as reliable providers in their industry, enhancing customer trust and satisfaction, and potentially leading to increased business and a competitive advantage.
ISO 13485:2016 is a standard that specifies requirements for a quality management system for the design and manufacture of medical devices. It includes requirements for how companies should design, implement, maintain and improve their quality management system to ensure that their medical devices are safe and effective. Test results can include information on how well the quality management system is functioning, how well it is being followed, and how effective it is in preventing defects. The standard also includes requirements for how the company should document and record their quality management system performance and continuously improve it.
Test results
Medical Management PassedThe ISO 13485:2016 standard is specifically tailored for medical device manufacturers and aims to ensure the quality and safety of medical devices throughout their production and lifecycle. A test result of Passed in the context of the ISO 13485:2016 standard signifies that the medical device manufacturing management system under assessment has successfully met all regulatory and safety requirements stipulated in the standard. This encompasses rigorous evaluations of the manufacturer's quality management system, including processes like risk management, regulatory compliance, and effective process control. The assessment involves auditing processes such as document review, facility inspection, and staff interviews, to verify the adequacy and effectiveness of the quality management system. Meeting the ISO 13485:2016 requirements assists manufacturers in achieving and maintaining regulatory compliance and ensures that their products consistently meet user needs and applicable regulatory standards, which is crucial for entry and continued presence in global markets.
CE Marking is a label that shows a product meets certain safety and environmental standards set by the European Union. To get the CE Marking, a company must test and certify their product meets these standards. CE Marking is required for many products sold in the EU, including electronics, machinery, toys and medical devices. It helps ensure that products are safe for consumers and the environment, and allows for easy trade within the EU.
MD stands for "Medical Device." It refers to any instruments, apparatus, machines, implants, or other similar or related articles that are intended to be used for human beings for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease, injury or disability. In Europe, a MD Label is a special label that must be on all Medical Devices that are sold or used in the European Union (EU). The label must include information about the product, such as the name of the manufacturer, the intended use of the product and CE mark. To be able to sell or use a Medical Device in the EU, the device must meet certain standards and requirements set by the European Union and notified body.
Medicom delivery terms
Free delivery when you order more than 150,00 € from Medicom
Supplier shipping fee 6,33 €
Brand minimum 200,00 €
Price available on request
Shipping fee is 6,33 € for orders under 150,00 €
A carton contains 12 packages (600 pieces)
Need larger quantities?
Other products you may like
Recently viewed
Need help?
Get help from our experts
Other products you may like
Similar products you may like
Recommended for you
Medicom
Delivery time: 5 business days
Orders from 200,00 €
Supplier shipping fee 6,33 €
Free shipping on orders over 150,00 €



Find +150,000 products from hundreds of brands
Autonomous sourcing platform
The most efficient way to source and order supplies for your operations
Sourcing
Ordering
List products you’re looking for and we’ll find the best products and prices for you – all for free.
Need help?
Get help from our experts