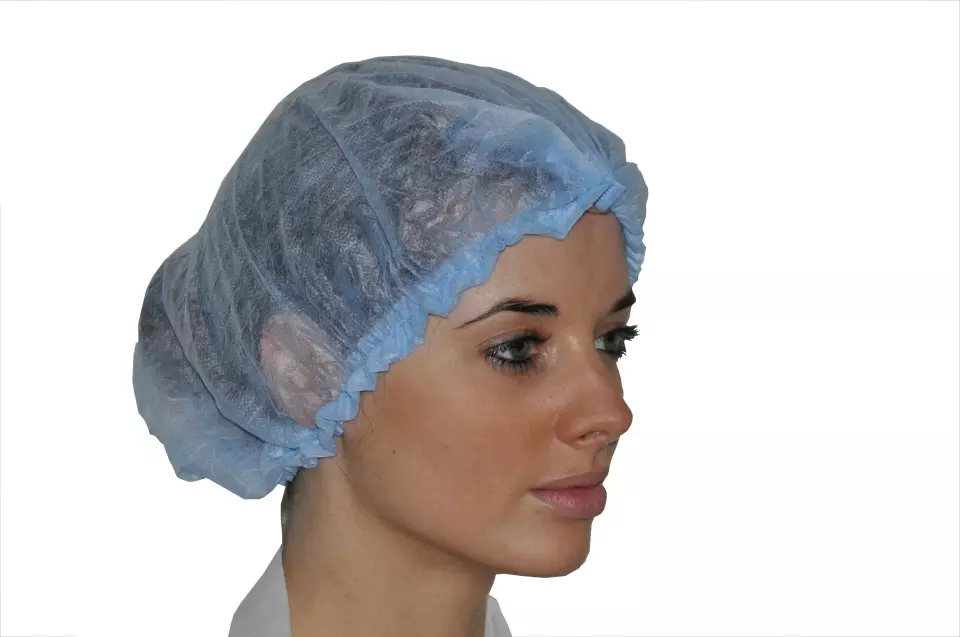
Kolmi PP Single Layer Elastic Bouffant Clip Cap, 10 g/m², Blue
4.3 / 5
Product description
Medical-grade bouffant clip featuring spunbonded non-woven polypropylene construction with ultrasound bonding technology. This latex-free head covering offers superior breathability and durability while providing a comfortable, stretchy fit for all hair types. Certified as a Class I Medical Device under EU Regulation 2017/745, it's specifically designed for healthcare environments.
Product Features:
- 100% polypropylene spunbonded non-woven material
- Ultrasound bonding assembly
- Latex-free synthetic elastic hem
- Highly breathable construction
- Universal stretch capability
Technical Details:
- Soft yet resistant non-woven material
- Single elastic design
- MD Class I certification
- CE marked
Recommended Applications:
- Healthcare facilities
- Hospital environments
- Medical procedures
Standards:
- EU Regulation 2017/745 compliant
- Medical Device Class I
- CE certification
- Water Resistance
Standards and labels
Medicom delivery terms
Free delivery when you order more than 150,00 € from Medicom
Supplier shipping fee 4,74 €
Brand minimum 200,00 €
Price available on request
Shipping fee is 4,74 € for orders under 150,00 €
A carton contains 12 packages (1 800 pieces)
Need larger quantities?
Other products you may like
Recently viewed
Other products you may like
Similar products you may like
Autonomous sourcing platform
The most efficient way to source and order supplies for your operations