
Features You'll Love
Filter Type · Type IIR
Indicates the level and type of filtration provided, determining what contaminants the respirator protects against and the percentage of particles filtered.

Mask Shape · Fold Flat
Respirator Style · Half Face
Determines the physical form of the mask, affecting comfort, breathing space, storage convenience, and how well it seals against your face.
Determines the coverage area and design of the respirator, such as full face or half face, affecting protection level and compatibility with other safety equipment.

Strap Type · Earloops
The method used to secure the respirator to your face, affecting comfort, fit, and ease of putting on and removing the mask.
144,98 €
Price per 40 packages (2 000 pcs)
0,072 € / piece
Shipping fee is 7,95 € for orders under 600,00 €
Features You'll Love

Filter Type · Type IIR
Indicates the level and type of filtration provided, determining what contaminants the respirator protects against and the percentage of particles filtered.

Mask Shape · Fold Flat
Respirator Style · Half Face
Determines the physical form of the mask, affecting comfort, breathing space, storage convenience, and how well it seals against your face.
Determines the coverage area and design of the respirator, such as full face or half face, affecting protection level and compatibility with other safety equipment.

Strap Type · Earloops
The method used to secure the respirator to your face, affecting comfort, fit, and ease of putting on and removing the mask.
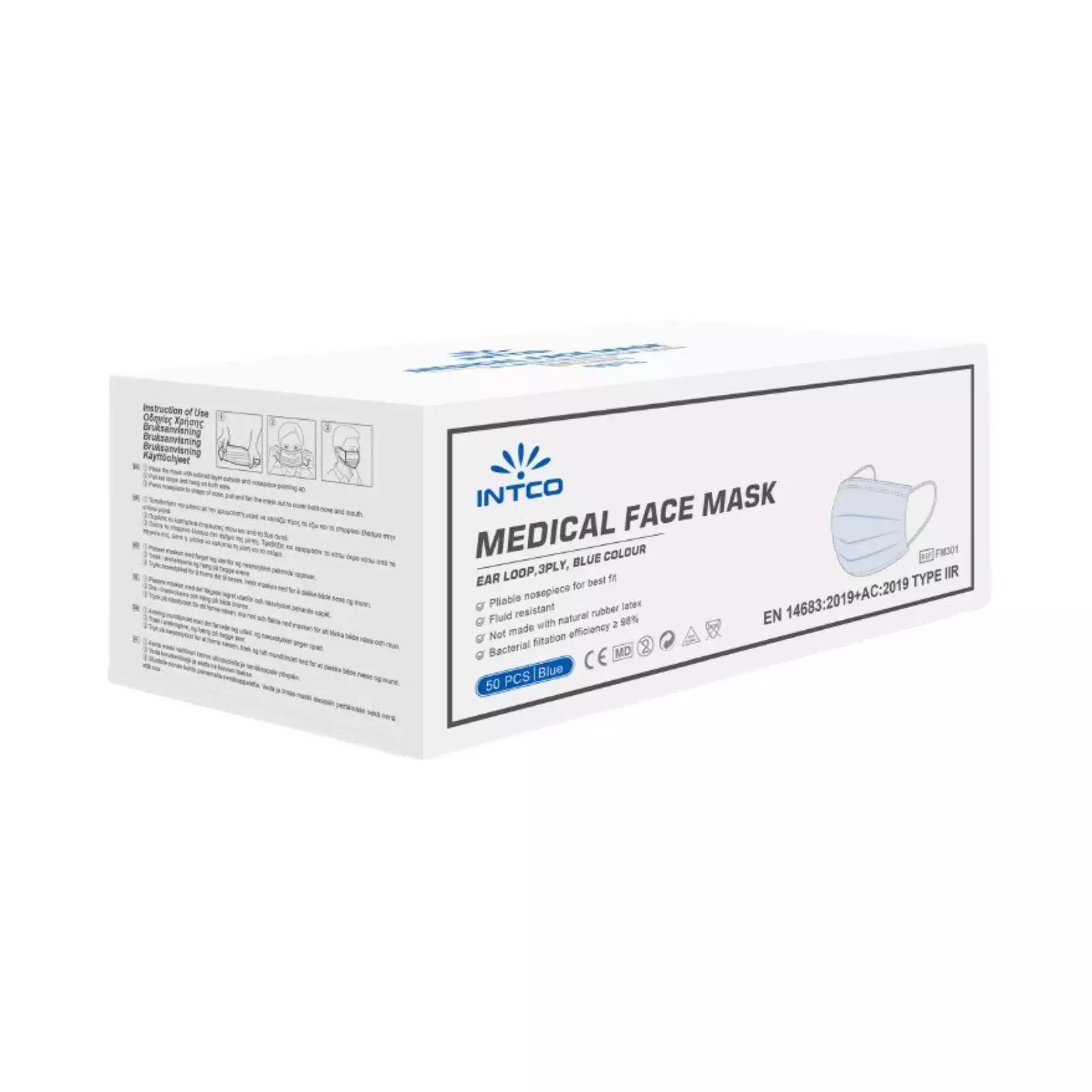
Product description
Disposable medical/surgical 3-layer face mask of superior quality. Skin-friendly fabric, elastic ear loops and adjustable nose bridge for good fit and tightness. Ultra-sonic weld secures strong construction and durability. Bacterial filtration efficiency above 99.8%, with a very high splash and fluid resistance.