Product description
The product description has not been specified
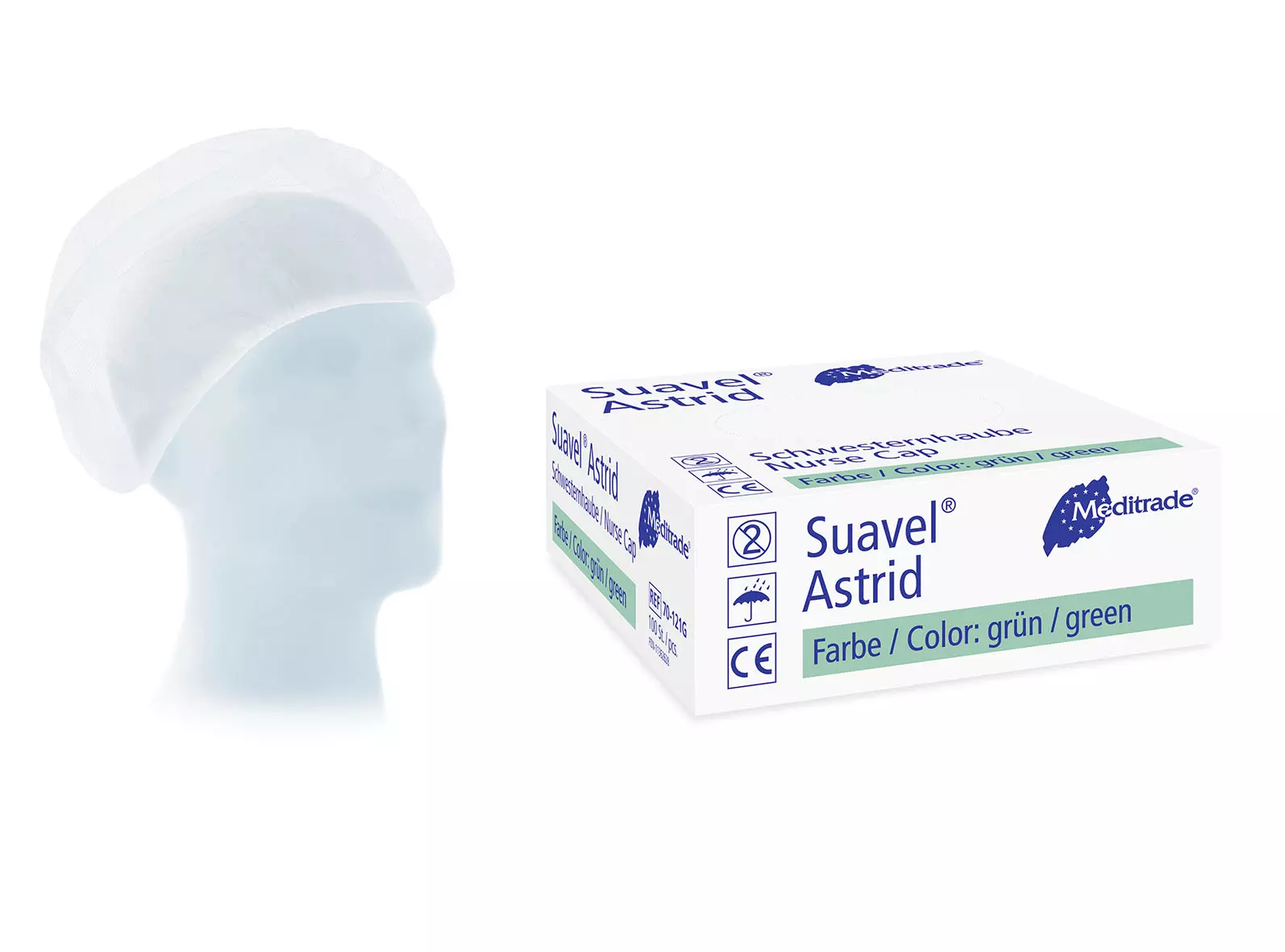
About Disposable Surgical Cap
Disposable Surgical Caps provide essential hygiene protection in medical environments by preventing hair contamination during procedures. Made from lightweight, breathable materials with secure elastic bands, these single-use caps help maintain sterile conditions while offering comfortable wear throughout long procedures.
- Medical Protection
Standards and labels
Meditrade delivery terms
Free delivery for all Meditrade products
Meditrade
Meditrade logo
Suavel® Astrid XL Nurse and Patient Cap, 1 000 pieces
Meditrade
Suavel® Astrid XL Nurse and Patient Cap, 1 000 pieces
Meditrade logo
5 / 5
70,07 €
77,89 €
Price per 10 packages (1 000 pcs)
0,07 € / piece
Estimated delivery: Tue Jan 13
Choose color
Minimum quantity: 3 cartons
Free delivery