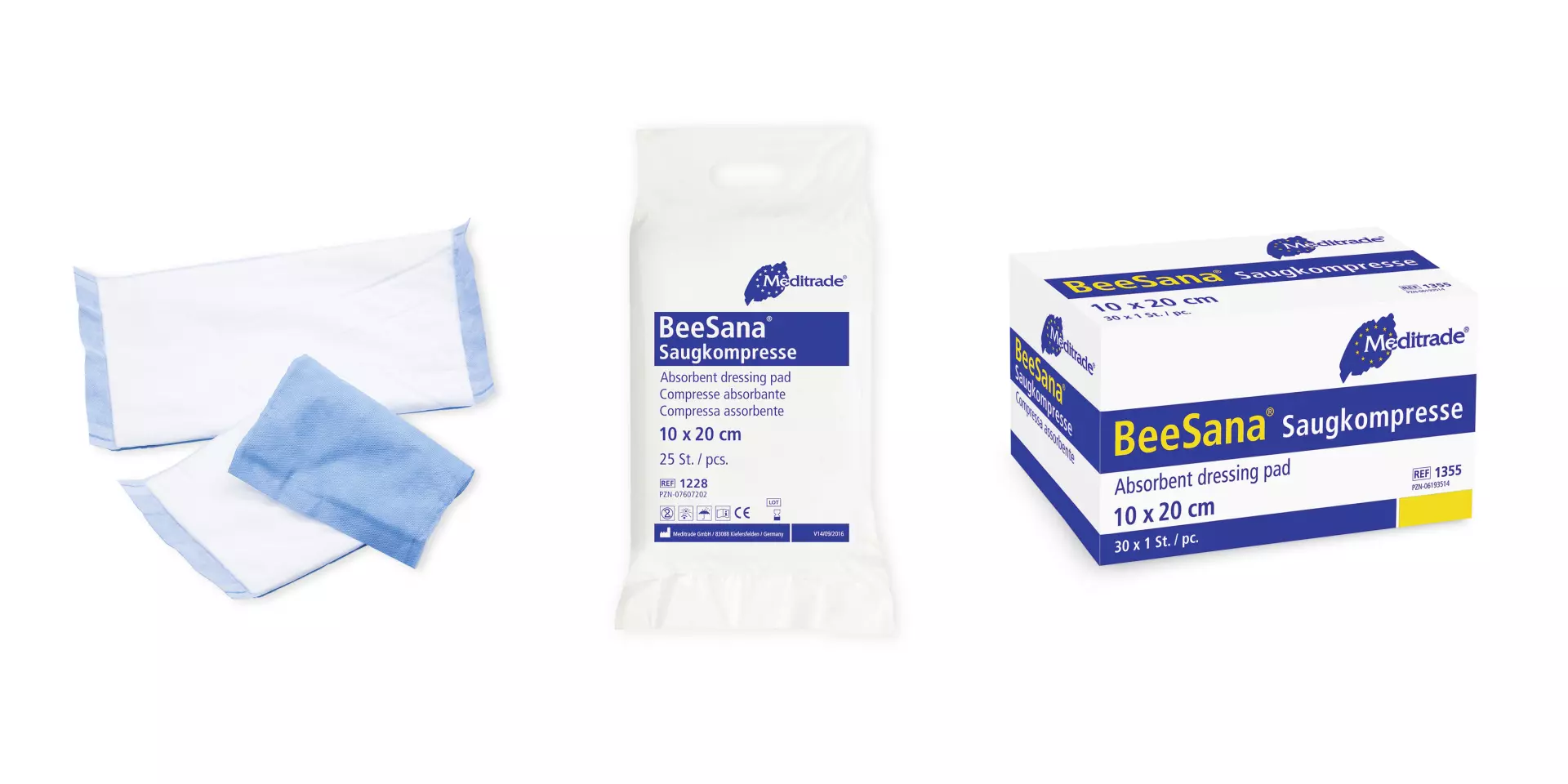
Features You'll Love
Container Style · Single Use Packet
Individually sealed for ultimate freshness and hygiene, perfect for convenient, on-the-go use.

Container Style · Box
Conveniently dispenses wipes from a sturdy box, keeping them protected and ready for use.
Meditrade
Meditrade logo
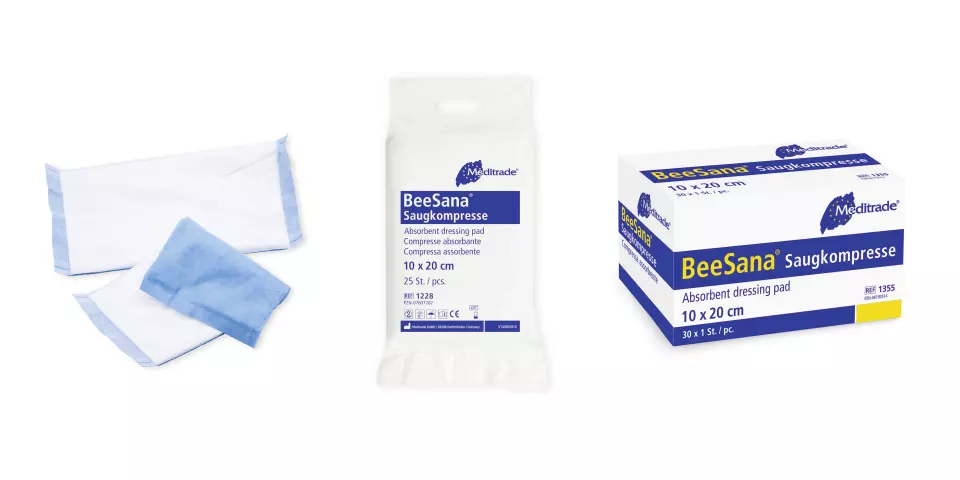
BeeSana® Absorbent Dressing Pad, Non-Sterile, 10 x 20 cm, 200 pieces
Meditrade
Meditrade logo
5 / 5
24,37 €
26,98 €
Price per 8 packages (200 pcs)
0,12 € / piece
Minimum quantity: 8 cartons
Total: 194,97 €8 cartons (1 600 pieces)
Shipping fee is 9,46 € for orders under 220,00 €
Features You'll Love

Container Style · Single Use Packet
Individually sealed for ultimate freshness and hygiene, perfect for convenient, on-the-go use.

Container Style · Box
Conveniently dispenses wipes from a sturdy box, keeping them protected and ready for use.
Product description
The product description has not been specified